
Powered By




The U.S. Food and Drug Administration (FDA) has cleared the MiniMed 780G automated insulin delivery (AID) system for use in adults with type 2 diabetes who require insulin. The system, already available to people with type 1 diabetes, combines an insulin pump with a continuous glucose monitor (CGM) to automatically adjust insulin delivery throughout the day.
Sign up to view the results!
 Continue with Facebook
Continue with your email
Continue with Facebook
Continue with your email
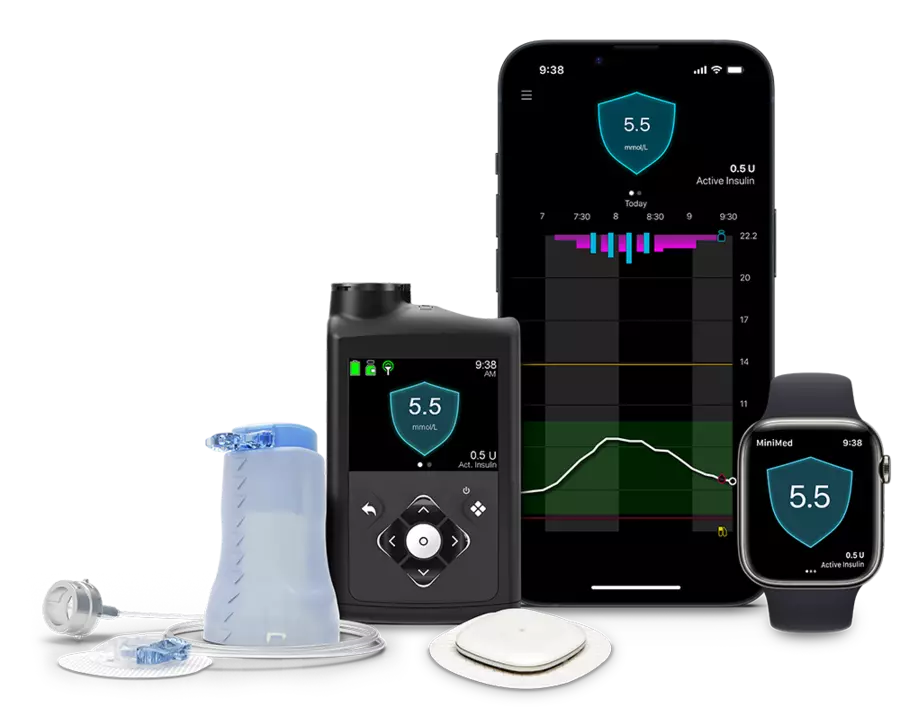
The MiniMed 780G system includes two main parts:
Together, these components form an automated insulin delivery system — sometimes called a “closed-loop” or “smart” insulin delivery system.
The CGM continuously tracks glucose levels and sends that information to the pump. The pump then uses this data to automatically adjust background (basal) insulin delivery, and it can also give correction doses when glucose levels rise above target. These automatic adjustments are designed to help reduce daily management tasks and keep glucose levels within range.
Unlike traditional insulin pumps, which require frequent manual dose changes, the MiniMed 780G responds to glucose trends in real time. Users still need to enter carbohydrate counts before meals, but the system’s built-in Meal Detection technology can help correct for meals that are delayed, unannounced, or underestimated.
Before the FDA approval, several studies examined how well AID systems work for people with type 2 diabetes.
In a 90-day trial of 95 adults with insulin-requiring type 2 diabetes, participants using the MiniMed 780G experienced an average drop in A1c (a measure of long-term blood sugar control) from 7.9 percent to 7.2 percent. Time spent in the target glucose range increased from 72 percent to 81 percent, with very low rates of hypoglycemia (dangerously low blood sugar).
Another larger study with 236 participants showed similar results: A1c decreased from 7.7 percent to 6.9 percent, and time in range rose from 76.4 percent to 84.9 percent.
Separately, a randomized controlled trial found that adults with insulin-treated type 2 diabetes using AID systems achieved significantly better glucose control than those using CGM alone. The AID group’s A1c fell by an average of 0.9 percentage points compared with 0.3 points in the control group, and participants spent more time in range with minimal hypoglycemia.
The FDA’s approval expands access to AID technology for people with type 2 diabetes who use insulin — a group that has historically relied on multiple daily injections or standard pump therapy.
For people managing type 2 diabetes, this technology could reduce the stress of frequent glucose checks, improve consistency in blood sugar control, and lessen the risk of complications linked to high or low glucose levels. They also have generally minimal side effects, such as mild skin irritation and itching at the infusion or sensor site.
Still, AID systems require training, attention to calibration, and coordination with healthcare professionals to ensure safe and effective use.
As new devices become available, access, affordability, and insurance coverage remain important considerations. People interested in AID systems should talk with their doctor about whether this type of technology fits their health needs and daily routine.
Learn more about the pros and cons of insulin pumps for type 2 diabetes.
On DiabetesTeam, people share their experiences with type 2 diabetes, get advice, and find support from others who understand.
Have you tried or are you considering an automated insulin delivery system? Let others know in the comments below.
Get updates directly to your inbox.



How Much Does It Cost??
 Continue with Facebook
Continue with your email
Continue with Facebook
Continue with your email
Become a member to get even more


This is a member-feature!
Sign up for free to view article comments.
A DiabetesTeam Member
I think I could benefit with that system
We'd love to hear from you! Please share your name and email to post and read comments.
You'll also get the latest articles directly to your inbox.